In many production environments, calibration certificates are seen as optional paperwork. Internal labs often run tests using their own machines, assuming accuracy by default. This mindset is common where companies aim to cut costs or avoid downtime.
But skipping proper calibration creates silent risks. Measurements drift. Tolerances tighten. One missed verification can lead to failed audits, rejected batches, or even lost contracts.
If a customer asks for traceable results and you can’t provide proof, the deal is gone. If a regulator inspects your equipment and finds outdated documents, your operations stop. These aren’t rare scenarios. They happen more often than most teams expect.
That’s why valid calibration isn’t just a box to tick. It’s a direct part of quality, compliance, and business continuity.
What Is a Calibration Certificate and Why It Matters
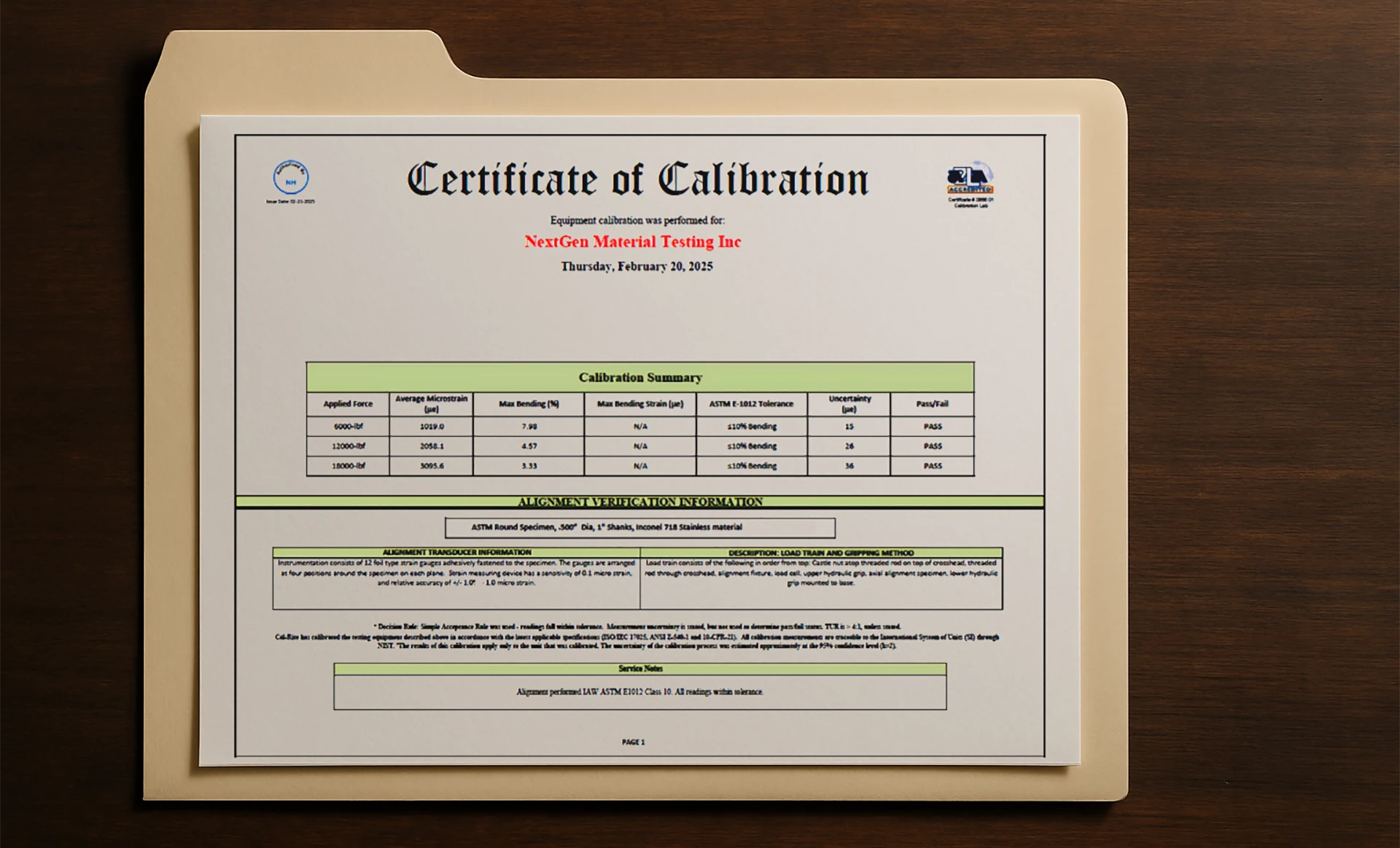
A calibration certificate is an official document that confirms a measuring instrument has been tested against recognized standards. It shows the device’s current condition and whether it performs within required tolerances.
This certificate is not a formality. It connects each measurement to a verified reference. That’s what allows test results to be trusted by auditors, regulators, and clients.
A proper certificate includes:
- Model and serial number of the instrument
- Measurement results before and after adjustment (as-found / as-left)
- Stated measurement uncertainty
- Calibration conditions: temperature, humidity, and environment
- Traceability chain to national or international standards
- Calibration date and next due interval
These details prove that the equipment is accurate, traceable, and ready for certified use. In production and testing labs, such documentation supports quality management, audit readiness, and technical reliability.
Why Calibration Certificates Are Non-Negotiable
Without calibration, measurement is guesswork. You may think your test press is within limits, but unless it’s certified, there’s no proof. A small drift in load cell readings, just 3%, can result in a weak part being approved and shipped. The consequences often appear later, at the worst moment.
A valid calibration certificate confirms the equipment was tested, adjusted, and verified against traceable standards. It proves that results are not only consistent, but measurable in an objective and standardized way.
This is essential in three scenarios:
- Quality control. Certificates show your process is under control. They confirm your team doesn’t rely on assumed values or outdated tolerances. For clients, this is a sign of technical discipline and transparency.
- Regulatory compliance. Standards like ISO 9001 and ISO/IEC 17025 require documented proof that equipment is calibrated. Without this, audits are failed. Equipment records must show traceability and acceptable uncertainty levels.
- Risk management. Instruments that drift introduce error. Over time, that error compounds: invalid test results, rejected batches, repeat inspections. A missed calibration leads to costs far greater than the certificate itself.
Accuracy Is Not Permanent
All measurement tools drift. Load cells, temperature sensors, even control modules lose precision with time. It doesn’t happen overnight, but it happens.
At a composites plant, a press had been running without calibration for nine months. The force reading was off by 2.5 percent. Around 8 percent of the batch was under-pressed and failed mechanical tests. The team had to reprocess the material and restart the test cycle. That meant lost time, wasted labor, and delayed delivery.
This isn’t rare. In any lab or production setting, even a small shift in measurement can break the quality loop. That’s why calibration isn’t just verification; it’s protection. It catches invisible drift before it turns into visible failure. It keeps data stable and traceable. It lets the equipment stay in use, not in quarantine.
Compliance with Standards and Regulations
 International regulations across aerospace, pharmaceuticals, automotive, and materials testing explicitly require documented calibration. These requirements are not advisory — they are built into most quality frameworks.
International regulations across aerospace, pharmaceuticals, automotive, and materials testing explicitly require documented calibration. These requirements are not advisory — they are built into most quality frameworks.
Key standards include:
- ISO 9001 – quality process validation
- ISO/IEC 17025 – laboratory competence and traceability
- ASTM E4, E18 – calibration protocols for test machines
A valid calibration certificate from an accredited lab confirms that the equipment has been tested under recognized procedures, using traceable reference instruments, and that measurement uncertainties have been evaluated and recorded.
Standards such as ISO 9001 and 17025 directly state that all equipment used for inspection and testing must be calibrated on a defined schedule, with documented results and traceable chains back to national or international references. During internal and third-party audits, this documentation is checked line by line. A missing or vague certificate can result in nonconformance, production hold, or failed recertification.
Regulatory bodies in specific sectors impose even stricter rules. The FDA in pharmaceuticals, the EASA in aviation, and national certification agencies in construction or defense all require proof of valid calibration performed by a qualified provider. Failure to present a valid certificate during regulatory checks may lead to financial penalties, lost contracts, or legal consequences.
The competence level of the calibration lab itself also matters. ISO/IEC 17025 is the global benchmark for technical credibility. Accredited labs undergo periodic audits, prove their measurement capability, and operate under strict metrological supervision. Calibration outside of this framework often lacks the necessary traceability details. Some providers skip uncertainty evaluation or omit environmental conditions entirely, leaving the certificate incomplete and potentially invalid in regulated environments.
Risks and Consequences of Missing Calibration Certificates
The absence of valid calibration certificates introduces measurable risks into production, testing, and certification workflows. These risks affect product quality, operational continuity, and client relationships.
Measurements from uncalibrated instruments cannot be trusted. Even a minor deviation may lead to entire batches being approved incorrectly. This results in manufacturing defects, increased scrap rates, and repeated testing.
International standards such as ISO 9001, ISO/IEC 17025, and ASTM require traceable calibration records. Missing documentation may cause audit rejection and suspension of certification status.
Many industrial clients and government buyers require up-to-date calibration reports for all testing equipment. Lack of documentation is a common reason for failed supplier evaluations and terminated agreements.
Inaccurate measurements increase the probability of production errors. This leads to delays, recalls, material waste, and overtime costs. Unscheduled downtime caused by troubleshooting defective results further raises operational expenses.
A materials testing company bidding for a defense contract was excluded after the inspection team discovered that its drop-weight impact tester lacked a current calibration certificate. No equipment malfunction was reported, but the absence of traceable verification was sufficient grounds for disqualification.
Counterfeit and Invalid Calibration Certificates
A legitimate calibration certificate must be issued by a laboratory accredited to ISO/IEC 17025 or an equivalent recognized standard. Certificates from non-accredited sources are not accepted during audits or regulatory inspections.
A valid certificate must contain the following:
- Instrument identification: model and serial number
- Calibration data with uncertainty values
- Reference to traceable standards
- Certificate number and issuing date
- Authorized signature and laboratory accreditation ID
Accredited laboratories often include QR codes or registration numbers that allow clients to verify certificates in official databases. Absence of such mechanisms may indicate a document is not authentic.
Using forged or unverifiable certificates carries the same consequences as having no calibration at all. These include audit rejection, loss of certifications, contract cancellations, and potential legal exposure.
Always validate calibration certificates by contacting the issuing laboratory directly or using its public verification system. Any doubts about authenticity must be resolved before the equipment is approved for use in regulated environments.
Calibration Certificates: A Business and Compliance Obligation
Calibration certificates are not bureaucracy: they are the foundation of operational stability. They confirm measurement accuracy, international compliance, and readiness for any audit. Without valid certificates, quality control fails, orders fall through, and businesses lose their competitive position.
NextGen Material Testing has been supplying a wide range of material testing equipment for many years, including presses, hardness testers, and systems for soil and rock analysis. All our equipment is delivered with valid calibration certificates.
In addition, we offer dedicated services for calibration, accreditation, and certification, both for our own systems and for third-party equipment. Working in compliance with ISO, ASTM, NADCAP, and other standards, we issue fully traceable documents. Our calibration partners are ISO/IEC 17025-accredited laboratories with recognized authority.
If you need certification, calibration, or an audit of your current systems, contact us. We’re ready to advise and provide a formal quotation.